Email: sales@qunkun.net |
TEL: +86 180 3242 3029
-
WIRE MESH WIRE NETTING
- WIRES AND NAILS
- FILTERS
-
SPECIAL ALLOY WIRE CLOTH
-
-
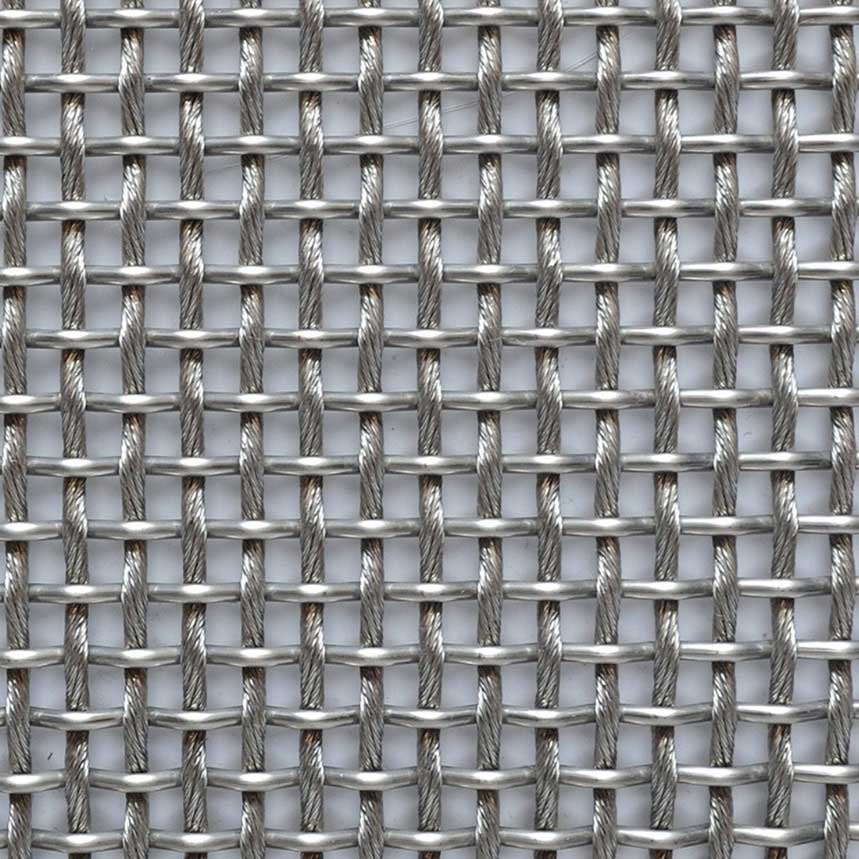
Plain Weaved Stainless Steel Wire Mesh

-
Duplex Stainless Steel Wire Mesh UNS 2304

-
Duplex Stainless Steel Wire Mesh UNS S31803(S32205)
.jpg)
-
Super Duplex Stainless Steel Wire Mesh UNS 32750

-
Dutch Weave Stainless Steel Wire Mesh

-
Reverse Dutch Weave Stainless Steel Wire Mesh

-
Twill Weaved Stainless Steel Wire Mesh

-
-
- DECORATIVE MESH
-
FENCING SYSTEMS
-
CHICKEN CAGE AND EQUIPMENT
- EXPANDED AND PERFORATED
.png)